Starting Thursday, the Food and Drug Administration will crack down on outsourcing facilities that make or distribute compounded alternatives to brand-name Ozempic and Wegovy.
State-licensed pharmacies and physicians were already stopped from doing so at the end of April, whereas the deadline for outsourcing facilities expires Thursday.
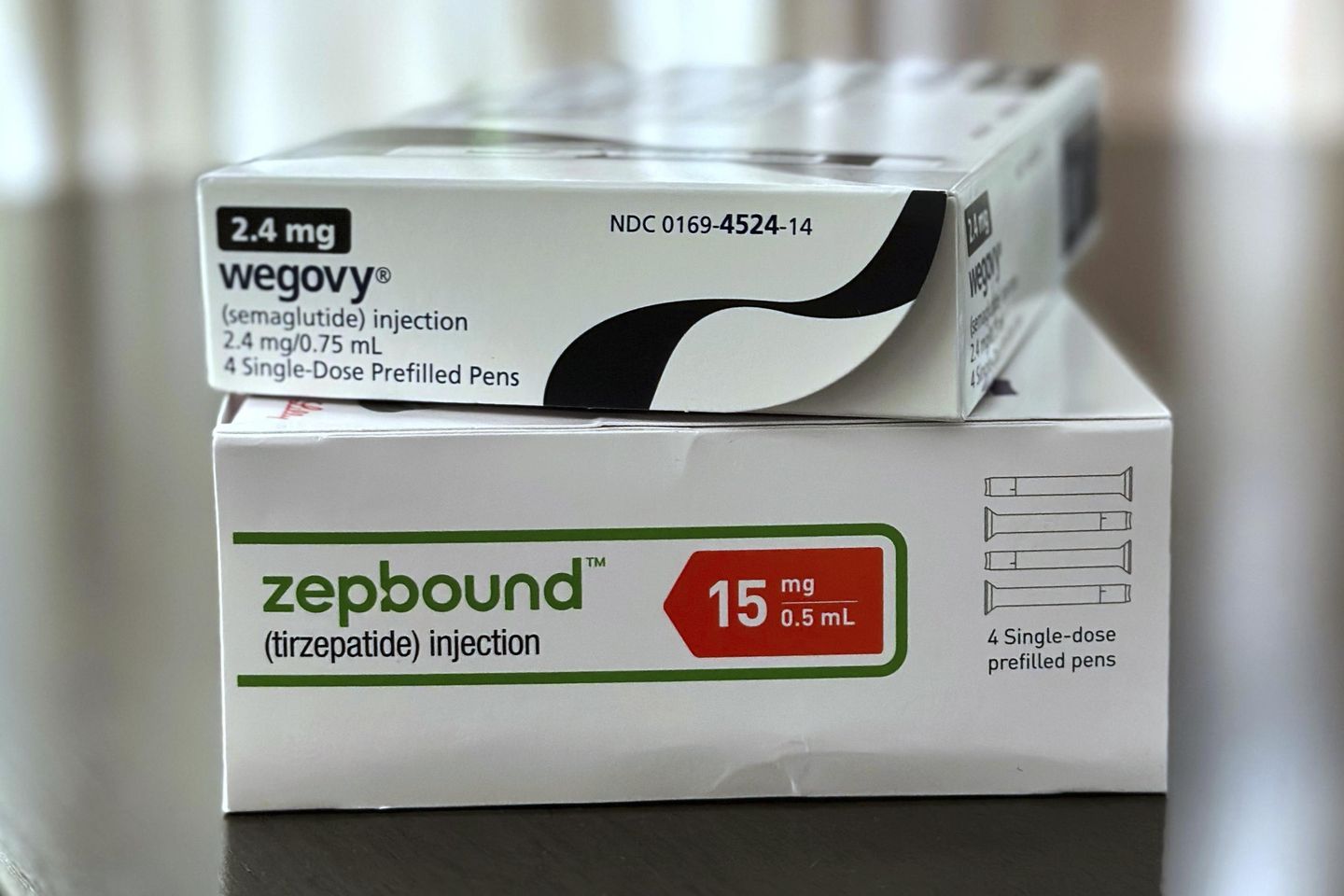
Ozempic and Wegovy are two injection products containing semaglutide produced by pharmaceutical giant Novo Nordisk. Ozempic is prescribed for Type 2 diabetes and Wegovy is for weight loss.
Semaglutide is a type of chemical known as a GLP-1 agonist, and physicians and outsourcing facilities were previously allowed to make and distribute compounded, off-brand versions of semaglutide products due to shortages.
Since the shortages are done, the FDA is no longer allowing compounding, it has explained on its website.
Another GLP-1, tirzepatide, is distributed by pharmaceutical company Eli Lilly under the names Mounjaro for diabetes and Zepbound for weight loss. The deadline for state-licensed pharmacies and physicians who compounded tirzepatide passed in February, while the deadline for outsourcing facilities passed on March 19.
The compounded pharmaceuticals industry contends that, contrary to what the FDA has said, both chemicals are still in shortage and making compounded versions of them should still be allowed.
Outsourcing Facilities Association Chairman Lee Rosebush told CNN he has “provided information to the administration … that these products – both GLP-1s – are still in shortage. What I’m afraid of happening at the end of this week when the deadline hits is that patients and providers won’t have access to the medications they need, and they will be financially impacted as they move forward because of this.”
Telehealth company Noom plans to provide alternative doses of compounded semaglutide after the deadline; the doses are in alternative amounts personalized for patients.
Noom CEO Geoff Cook told Reuters that “there is a personalized, and there has always been a personalized, exception.”
The makers of the brand-name medicines are strongly against the continued production of compounded alternatives and disagree that there is a personalization exemption when it comes to compounded drugs.
“Anyone continuing to sell mass-compounded tirzepatide, including by referring to it as ‘personalized,’ ‘tailored’ or something similar, is breaking the law and putting patients at risk,” Lilly said in a statement to CNN.
A Novo Nordisk spokesperson told Reuters that “compounders cannot evade federal compounding laws by selling knockoff semaglutide drugs with manipulated, unnecessary, and pretextual changes to doses and ingredients.”
Some medical professionals are also concerned about the quality of compounded medicines.
“We have been conducting a reckless national experiment with compounded new weight-loss drugs,” Dr. David Kessler, former FDA commissioner, said at a congressional hearing in April.












