I have been highly critical of the British Medical Journal, with good reason. It publishes some good research, and way too much political tripe.
This story falls more into the former category than the latter.
The US Food and Drug Administration says that it takes conflicts of interest seriously. But financial entanglements with the drug industry are common among its leaders.
Peter Doshi reports
🔗 https://t.co/LM9hJdDo1F pic.twitter.com/HatkDZyvAM— The BMJ (@bmj_latest) May 8, 2024
Anybody who has taken a serious interest in the relationship between the Food and Drug Administration and the pharmaceutical and medical device industries is struck by an obvious fact: the revolving door between the regulators and the regulated is utterly corrupt.
The phenomenon is called regulatory capture. When this happens, as it often does, the regulators see their own interests as more aligned with the industry than with the general public. It happens much more often than you would think or hope. I once heard a story from a friend who worked on Capitol Hill about a Senate researcher who helped write tax laws.
He put in a provision that was so complicated that nobody could make heads or tails of it, then went into business as a consultant who explained what it meant to people.
He made bank.
Regulatory capture is similar to this. The regulators become “experts” on how the system works, making themselves extremely valuable to the regulated. No industry would ignore the value of having especially good relations with the people on the other side of a negotiating table.
It’s a match made in heaven, except for you and me. We are the rats experimented upon.
There are, of course, ethics rules that are supposed to prevent conflicts of interest, and when regulators are working in government they are not allowed to invest in companies they regulate.
The US Food and Drug Administration says that it takes conflicts of interest seriously. But financial entanglements with the drug industry are common among its leaders. Peter Doshi reports
At his public confirmation hearing in late 2021, Robert Califf, President Biden’s nominee to lead the US Food and Drug Administration (FDA), faced pointed questions about his financial relationships with industry.
Bernie Sanders, the senator from Vermont, asked, “At a time when the American people are outraged by the high cost of prescription drugs, deeply disturbed about what happened with Purdue and Oxycontin, what kind of comfort can you give to the American people when you have been so closely tied to the pharmaceutical industry yourself?” He added, “How can the American people feel comfortable you’re going to stand up to this powerful special interest?” Califf responded: “Senator Sanders, I have a history of doing that. But I’d also point out that this administration has the most stringent ethics pledge in the history of administrations.”
Califf did not earn Sanders’s vote, but he got the job. With it, the incoming FDA commissioner committed to sell his pharmaceutical stocks and sever his financial relationships with biotech companies such as Alphabet owned Verily Life Sciences, which paid Califf $2.7m as a senior adviser, according to his federal disclosure (see supplementary files on bmj.com).
Of course, when there is a revolving door, this is something of a scam. While it is true that having people with experience in the medical industries can be valuable for government regulators–they know the business, after all–the opposite is true as well.
And, of course, the friendlier the regulator is to an industry, the more valuable they become to those within it.
The year 2022 marked Califf’s second time leading the FDA, having previously served during the Obama administration’s final year. It was therefore his second time terminating a host of ties with the companies that the agency is meant to regulate, and his second time signing an ethics pledge.
The Trump administration, too, required that appointees sign an ethics pledge, committing not to lobby the agencies for five years after public service. But the requirement was rescinded on 19 January 2020, Trump’s last full day in office. In addition, the ban only applied to lobbying activities, not employment in general, and within three months of vacating the FDA’s top job, Scott Gottlieb, Trump’s first nominee who led the agency from 2017 to 2019, was nominated to Pfizer’s board of directors, subsequently gaining enormous public visibility through regular media appearances as a covid expert commentator. (While a medical student, Gottlieb interned at The BMJ as a Clegg scholar; he subsequently penned a number of BMJ news articles.)
The revolving door between the FDA and industry surprises few anymore, despite the widely acknowledged potential it has for undermining public trust in government. And stories about FDA commissioners’ heavy ties to industry have become commonplace: nine of the FDA’s past 10 commissioners went on to work for the drug industry or serve on the board of directors of a drug company.
This happens all the time, and bureaucrats like it that way. And, frankly, so do many lawmakers who can do the same sort of thing–build up good relationships with companies and retire later with a windfall.
In political science we call this the iron triangle, where the regulators, the industries, and the politicians all share a common interest that diverges from the public’s.
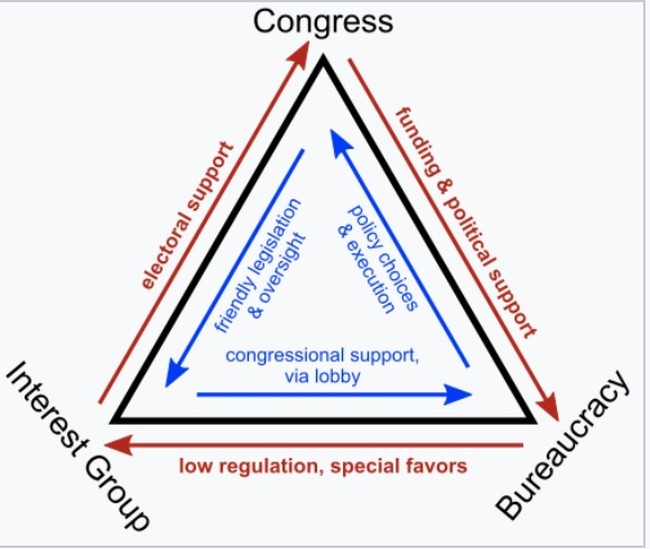
This is how you get unsafe and/or ineffective drugs through the system and how, I suspect, the jabs got out into the wild. Tens of billions of dollars are made based on these sorts of decisions.
Paxlovid is a great example. It has never been shown to be effective in the least, but the government spent zillions of dollars buying it and promotes the heck out of it as the money rolls into the coffers of Pfizer. It also has a pretty nasty side-effect profile.
New England Journal Paxlovid study: “no significant difference in the time to symptom alleviation” in vax
This trial ended >1.5 yrs ago. Why are the results only being released now??
After $20B taxdollars & a big campaign urging ppl <65 to take it. Amazhttps://t.co/WvGWtDCjbm
— Marty Makary MD, MPH (@MartyMakary) April 24, 2024
This is how it works, folks. Follow the money.












